Abstract
Introduction: Peripheral T-cell lymphomas (PTCLs) comprise a subset of blood cancers that are molecularly heterogeneous and often associated with poor survival. While brentuximab vedotin has shown efficacy in CD30-expressing lymphomas such as anaplastic large cell lymphoma, effective targeted therapies for PTCLs are lacking and this represents an unmet medical need.
CD6, an established surface marker for T cells, is a key regulator of T cell responses. CD6 has been suggested as a target for treating T cell-mediated autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis. We previously reported that CD6 knockout mice show reduced pathogenic T-cell responses and thus are protected against several models of autoimmune diseases. We also found that CD6 could be targeted therapeutically by anti-CD6 mAbs in these mouse models of autoimmune diseases. Although the distribution of CD6 and its role in regulating normal, non-malignant T-cells has been established, the expression of this marker on PTCL cells and its potential use as a selective targeted therapeutic agent for PTCLs has not been explored.
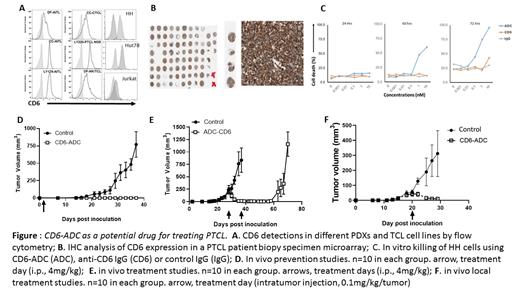
METHODS: To determine the expression profile of CD6 on the malignant T-cells, different specimens including established T-cell lymphoma/leukemia cell lines HH, MOLT-4, HUT-78 and JurkaT-cells, as well as PTCL patient-derived xenografts (PDX) were analyzed for CD6 expression by flow cytometry using UMCD6, a high-affinity anti-human CD6 mAb. Besides, a custom-made tissue microarray containing biopsy samples from different PTCL patients was also evaluated by immunohistochemical staining using the same mAb.
A CD6-targeted antibody-drug conjugate (CD6-ADC) was prepared by conjugating monomethyl auristatin E (MMAE), a potent antimitotic toxin successfully used in other FDA-approved ADCs, to UMCD6. Control non-binding ADC was prepared the same way using purified mouse IgGs. The potency of the CD6-ADC of killing PTCL was first evaluated in vitro using HH and HUT78 cells with trypan blue staining. For in vivo treatment studies, NSG mice were subcutaneously inoculated with HH cells, then treated with CD6-ADC or controls at different time points either systemically by intraperitoneal injections or locally by intratumor injections. Treatment efficacies were evaluated by measuring subcutaneous tumor volumes and detecting metastasized tumors in bone marrows, spleens and livers.
RESULTS: Our flow cytometric and immunohistochemical staining studies showed that most, if not all the specimens examined expressed high levels of CD6 on the surface, suggesting that CD6 could be a novel therapeutic target for this molecularly heterogeneous blood cancer. Besides, our anti-CD6 mAb, was quickly internalized by PTCL cells after binding to CD6 on their cell surface in an in vitro assay. In contrast to either the parental anti-CD6 mAb, or the non-binding control ADC, the CD6-ADC potently and selectively killed T lymphoma cells in vitro in a time- and concentration-dependent manner. It further prevented the development of tumors in vivo when given 1 day after PTCL cell inoculations. More importantly, systemic or local administration of CD6-ADC, but not the control ADC, significantly shrank existing tumors, reduced tumor metastases from the already developed tumors, and prolonged overall survival in the preclinical mouse model of PTCL without apparent adverse effects.
CONCLUSIONS: These results suggest that CD6 is a novel therapeutic target for PTCLs and provide a strong rationale for the further development of CD6-ADC as a promising therapy for patients with this aggressive blood cancer.
Hsi: AbbVie Inc, Eli Lilly: Research Funding. Lin: takeda Pharma: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal